Dementia Research

The Miller Lab studies how different proteins, including tau and TREM2, affect neurodegeneration. We employ RNA-targeted therapeutic strategies using antisense oligonucleotides to understand disease processes and develop therapies for dementias.
Antisense oligonucleotides for tau-related disorders and dementias
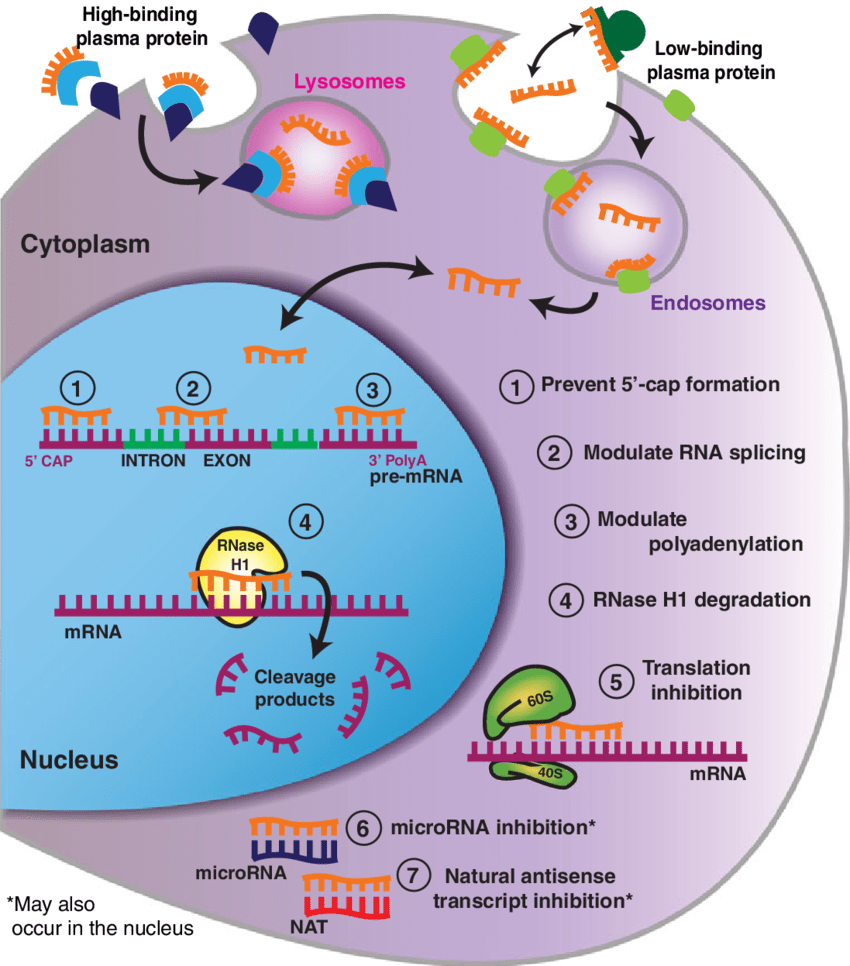
The Miller Lab is currently investigating the therapeutic ability of antisense oligonucleotides (ASOs) to target tau in dementias. ASOs are small DNA-like molecules that target specific genes to prevent the production of a protein or change the expression of protein isoforms. We have designed ASOs that either lower tau or direct alternative splicing of the MAPT gene and use these methods to interrogate various mechanisms of neurodegenerative disease.
We have previously shown that lowering tau protein in healthy adult mice can reduce seizure severity, suggesting tau plays an important role in modulating neuronal hyperexcitability (DeVos et al. 2013). Using genetic mouse models of dementia, we have also shown that lowering tau in the central nervous system can reverse mutant tau pathology, lessen hippocampal volume loss, prevent functional deficits, and prolong survival (DeVos et al. 2017). These data demonstrate that ASOs can be both safe and effective in vivo in models of tau-related disorders and dementia. Following this work, a tau-targeted ASO clinical trial for Alzheimer disease (AD) patients was launched (see Biogen press release). Our goal is to bring this novel therapeutic strategy to patients with tauopathies.
Tau isoforms in disease
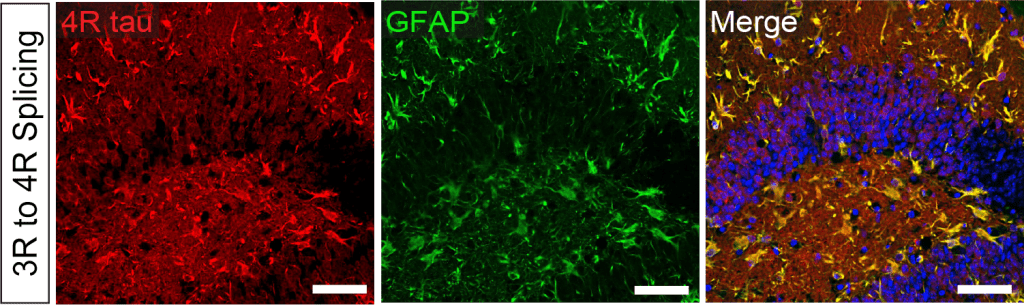
In addition, the Miller Lab is investigating tau isoforms, particularly 3R and 4R tau, which arise from alternative splicing of MAPT. Several tauopathies exhibit defects in tau splicing and/or selective deposition of 3R or 4R tau; however, the role of these specific isoforms in disease remains unclear. We used ASOs to manipulate 3R and 4R tau ratios, showing that greater 4R tau in mice increased phosphorylated tau deposition and functional deficits (Schoch et al. 2016). More recent data suggest that astrocytes are especially vulnerable to increased 4R tau, inducing a neurotoxic phenotype (Ezerskiy et al. 2022). These data suggest that targeting 4R tau and astrocytes may be an effective therapeutic strategy for 4R tauopathies.
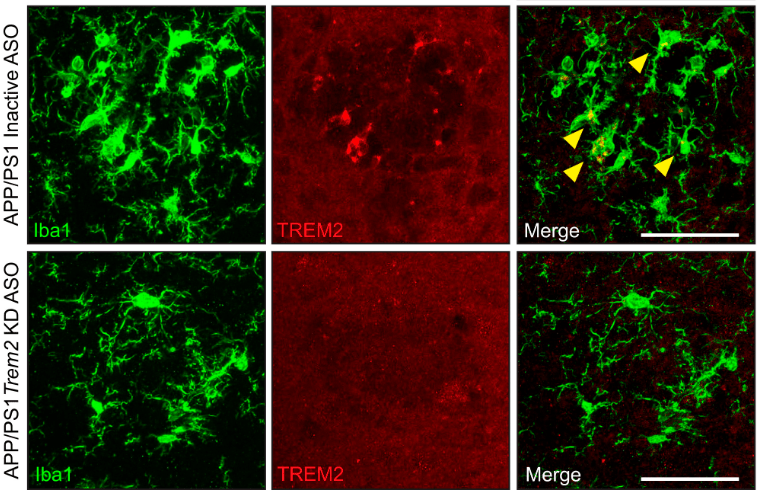
Targeting TREM2 to alter microglial responses
Variants within the gene TREM2 (triggering receptor expressed on myeloid cells 2) have known risk for AD presumably through expression of the TREM2 receptor on microglial cells within the brain. We have used an amyloid-depositing mouse model to test TREM2-lowering ASOs on amyloid plaque deposition and microglial responses to better understand how TREM2 mediates the interaction between microglia and AD pathology (Schoch et al. 2022). We continue to study and understand other targets for neuroinflammation that can be specifically altered using ASOs.
Autophagy/proteasome activation to promote tau clearance
ASO-mediated tau lowering can achieve remarkable reduction and reversal of tau pathology in mutant tau mice. The Miller Lab is currently investigating what protein degradation systems are involved in tau removal after ASO treatment and if these systems can be targeted to enhance the clearance of tau pathology.